The suspension polymerisation process is most widely used process to manufacture PVC.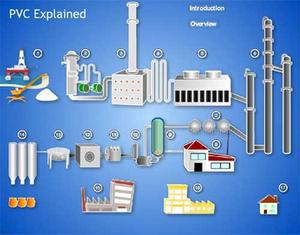
- First, the raw material VCM is pressurised and liquefied, and then fed into the polymerisation reactor, which contains water and suspending agents in advance. Through high-speed agitation within the reactor, small droplets of VCM are obtained.
- Next, the initiator for polymerisation is fed into the reactor, and PVC is produced by reaction under a few bar at 40 - 60°C. PVC obtained through suspension polymerisation is suspended in water as particles of 50~200 μm diameter (in slurry form).
- Thereafter the slurry discharged from the polymerisation reactor is stripped of residual monomer, dehydrated, dried and the particle size controlled by screening to yield PVC in the form of a white powder.
- The un-reacted VCM is entirely recovered through the stripping process, and after purification, recycled as raw material for reuse in this process. PVC resin produced via this ‘suspension’ process is referred to within the industry using the abbreviation S-PVC.
Emulsion polymerisation and bulk polymerisation are alternative, much less extensively employed, technologies to manufacture PVC.
Emulsion polymerisation produces finer resin grades having much smaller particles, which are required by certain applications. This type of resin is sometimes called ‘paste’ PVC and referred to within the industry using the abbreviation P-PVC to distinguish it from S-PVC.
Click the diagram for an interactive animation showing how PVC is produced. Once the diagram has loaded click any section to see part of that process or on the OVERVIEW to watch to all sequences.









